Sinopharm Vaccine Efficacy Lancet : Can I Get Astrazeneca Now And Pfizer Later Why Mixing And Matching Covid Vaccines Could Help Solve Many Rollout Problems
Vaccine efficacy against hospitalization was 79. The Lancet Interim analysis from phase 3 trial of nearly 20000 participants suggests efficacy of two-dose regimen of the adenovirus-based vaccine is 916 against symptomatic COVID-19 - trial.
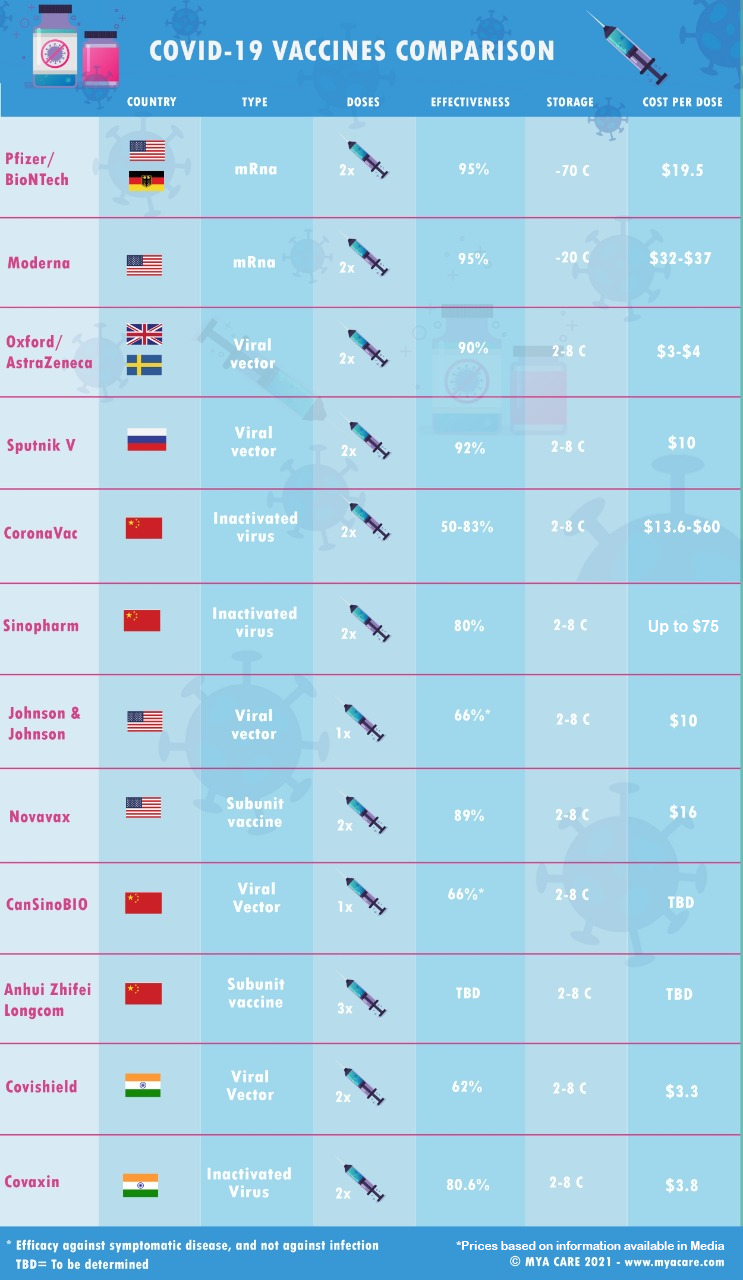
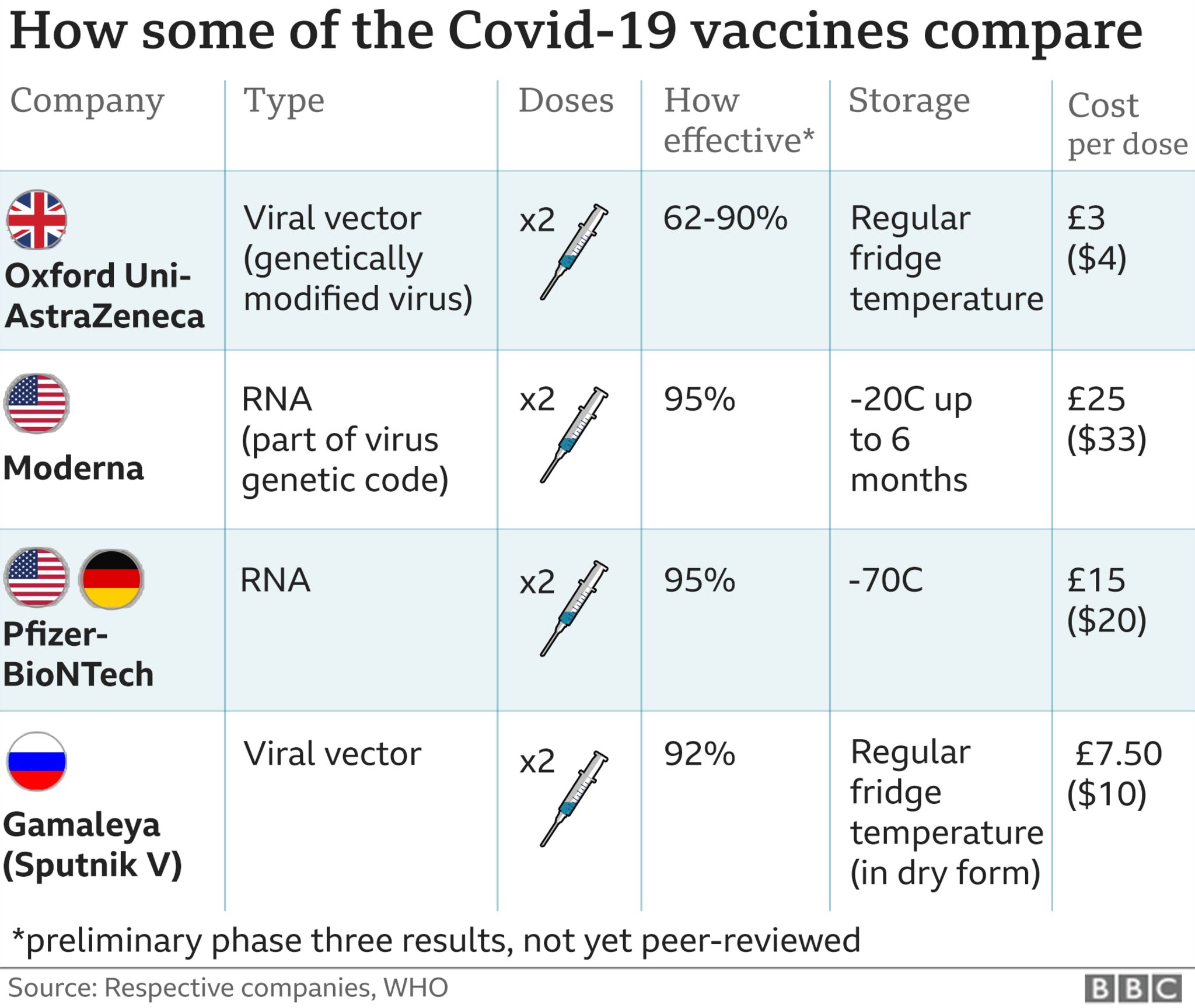
Comparison Of Covid 19 Vaccines Mya Care
The development of inactivated vaccines is a mature technology which is widely used for.

Sinopharm vaccine efficacy lancet. Women were underrepresented in the trial. The company has yet. We identified a phase 12 study of an inactivated SARS-CoV-2 vaccine BBIBP-CorV developed by Sinopharm Beijing China which showed that the vaccine was safe tolerable and immunogenic in healthy people in China.
More than 51 million doses of the jab have been. Design setting and participants. The WHO recommends the Sinopharm vaccine for people aged 18 years and older with a gap of 34 weeks between the two doses.
Among 11 303 volunteers screened between Sept 14 2020 and Jan 5 2021 10 218 were randomly allocated. Approximately 96 COVID-19 vaccines are at various stages of clinical development1 At present we have the interim results of four studies published in scientific journals on the PfizerBioNTech BNT162b2 mRNA vaccine2 the ModernaUS National Institutes of Health NIH mRNA-1273 vaccine3 the AstraZenecaOxford ChAdOx1 nCov-19 vaccine4 and the Gamaleya GamCovidVac Sputnik V vaccine. The ongoing COVID-19 pandemic warrants accelerated efforts to test vaccine candidates.
The UAE approved the vaccine developed by the Chinese state-owned Sinopharm on December 9 2020 UAE announced that the vaccine was 86 efficacious according to. However updated evidence is now available for several inputs ie rotavirus disease mortality rates rotavirus age distributions vaccine timeliness and vaccine efficacy by duration of follow-up new rotavirus vaccines have entered the market vaccine prices have decreased and. 30 Sinopharm announces that the vaccine has an efficacy of 7934 percent leading the Chinese government to approve it.
The AstraZeneca vaccine rounded out the bottom with 62 percent initial efficacy and a half life of 48 days though the Sinopharm vaccine fared even worse having just. The global health agency estimates overall vaccine efficacy to. Adenovirus-vectored vaccines RNA vaccines protein subunit vaccines and virus-like particle vaccines.
China-made coronavirus vaccines widely distributed despite efficacy concerns Sinovac published combined phase 1 and 2 results for its CoronaVac vaccine. To assess the efficacy and adverse event profile of the recombinant zoster vaccine in immunocompromised autologous HSCT recipients. We also identified a phase 2 clinical trial of another inactivated vaccine developed by Sinopharm Beijing China which showed the incidence of adverse reactions was 190 within 28 days after two doses of vaccine 5 μg in 05 mL of diluent in a day 0 and 21 vaccination schedule and.
The United Arab Emirates the first foreign country to approve a Covid-19 vaccine developed by Chinese. Sign up to myFT Daily Digest to be the first to know about Coronavirus treatment news. Further studies of the effectiveness of this vaccine for prevention of.
Two weeks after the second dose the differences in vaccine effectiveness by variant were more modest with the PfizerBioNTech jab offering 88. After exclusion of four participants from the vaccine group because of protocol deviations the intention-to-treat group consisted of 10 214 participants 6646 651 in the vaccine group and 3568 349 in the placebo group and the per protocol group consisted of 10 029. Real-world test-negative analysis in Bahrain based on 14 days post 2nd dose.
The safety and efficacy of some of these candidates have been shown in preclinical trials and the safety and immuno-genicity in clinical trials. The World Health Organization WHO recommends an interval of 3 to 4 weeks between doses. We aimed to assess the safety and immunogenicity of an inactivated severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 vaccine candidate BBIBP-CorV in humans.
Previous studies have found rotavirus vaccination to be highly cost-effective in low-income countries. The vaccine is given by intramuscular injection into the deltoid muscleThe initial course consists of two doses and there is no evidence that a third booster dose dose is needed. Phase 3 randomized observer-blinded study conducted in 167 centers in 28 countries between July 13 2012 and February 1 2017 among 1846 patients aged 18 years or.
17 British medical journal The Lancet featured a study about the efficacy of Sinovac Biotechs vaccine candidate based on initial clinical trials. The trial was not designed and powered to demonstrate efficacy against severe disease in persons with comorbidities in pregnancy or in persons aged 60 years and above. The median duration of follow-up available at the time of evidence review was 112 days.
The Lancet Child Adolescent Health. The Lancet Child. It found that the Chinese companys.
It was the first country in the 27-member bloc to approve Russias Sputnik V vaccine and is the only one to deploy Chinas Sinopharm.
Can I Get Astrazeneca Now And Pfizer Later Why Mixing And Matching Covid Vaccines Could Help Solve Many Rollout Problems

An Interactive Website Tracking Covid 19 Vaccine Development The Lancet Global Health
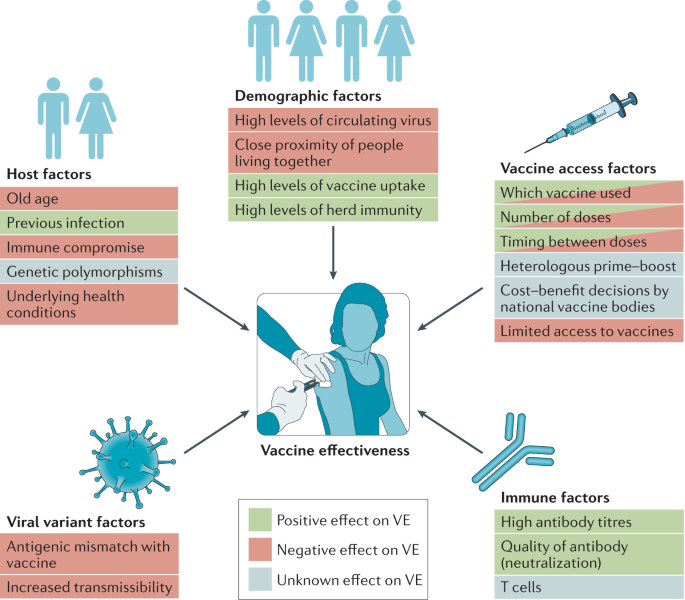
Progress Of The Covid 19 Vaccine Effort Viruses Vaccines And Variants Versus Efficacy Effectiveness And Escape Nature Reviews Immunology

Safety And Efficacy Of The Chadox1 Ncov 19 Vaccine Azd1222 Against Sars Cov 2 An Interim Analysis Of Four Randomised Controlled Trials In Brazil South Africa And The Uk The Lancet

Safety And Efficacy Of An Rad26 And Rad5 Vector Based Heterologous Prime Boost Covid 19 Vaccine An Interim Analysis Of A Randomised Controlled Phase 3 Trial In Russia The Lancet

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases
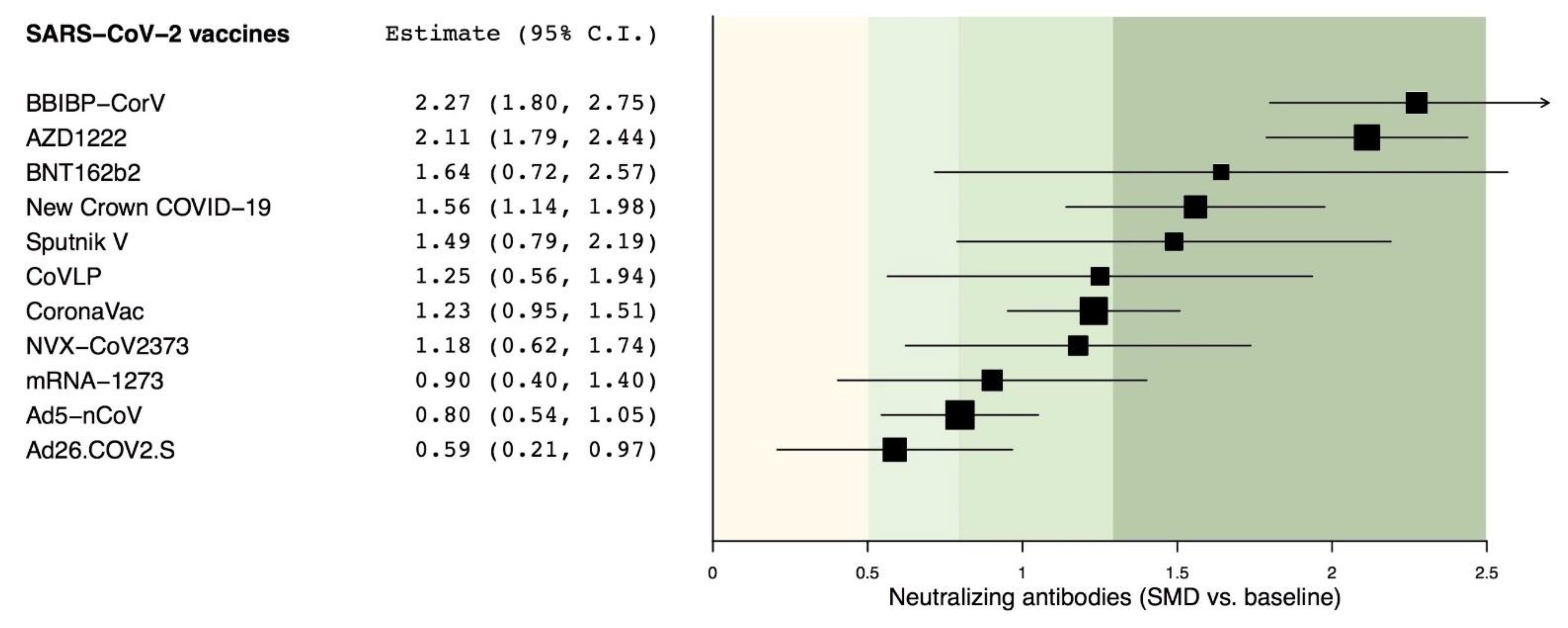
Vaccines Free Full Text Sars Cov 2 Neutralizing Antibodies A Network Meta Analysis Across Vaccines Html

Russia S Sputnik V Vaccine Is 91 6 Effective Lancet Study Hindustan Times
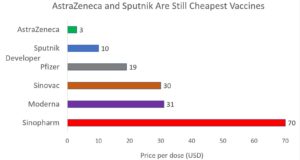
Publication Of Sputnik V Results Shore Up Prospects Of Elusive Trio But Answers Still Needed On China S Covid 19 Vaccines Health Policy Watch
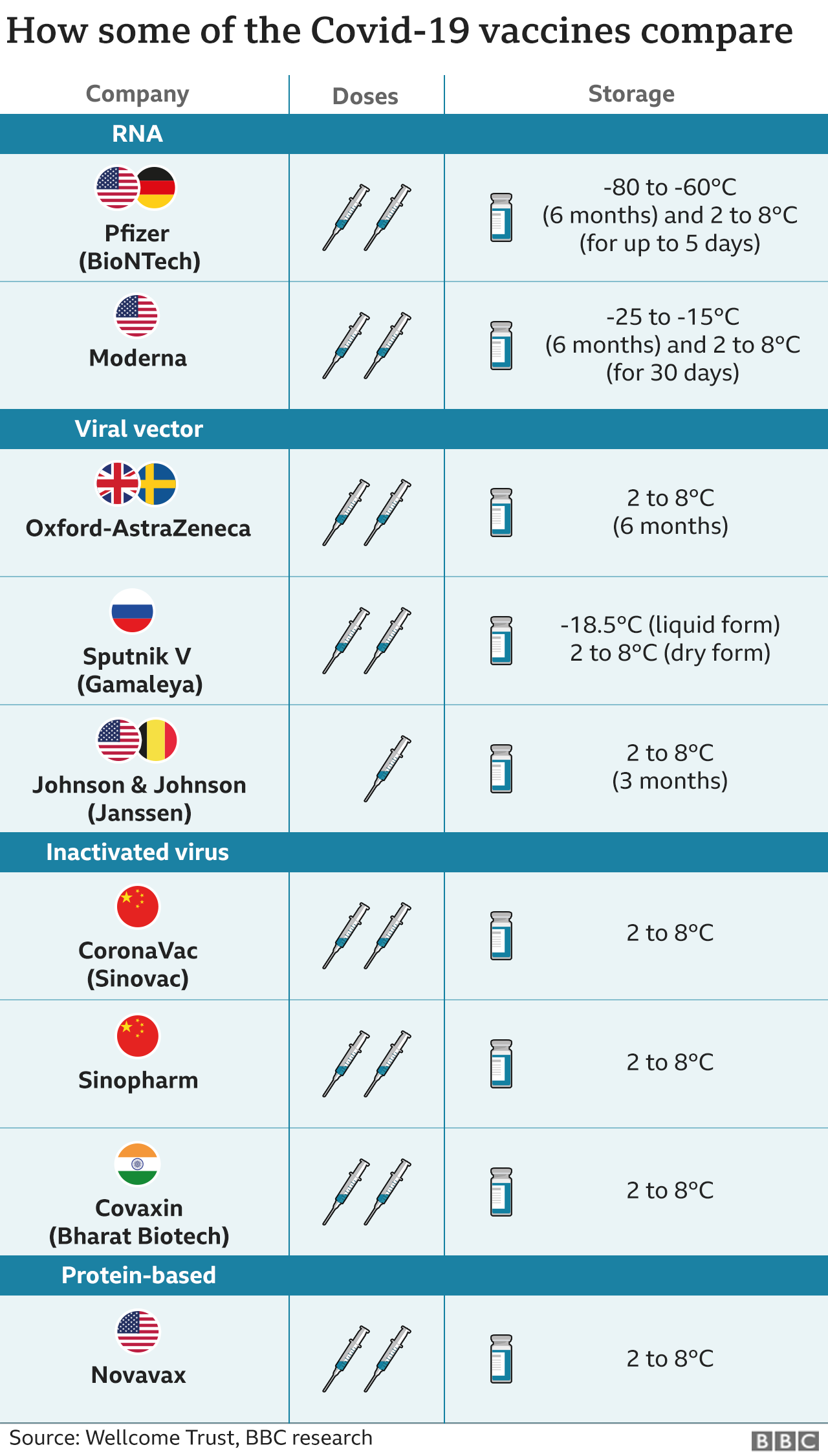
Sinopharm Chinese Covid Vaccine Gets Who Emergency Approval Bbc News
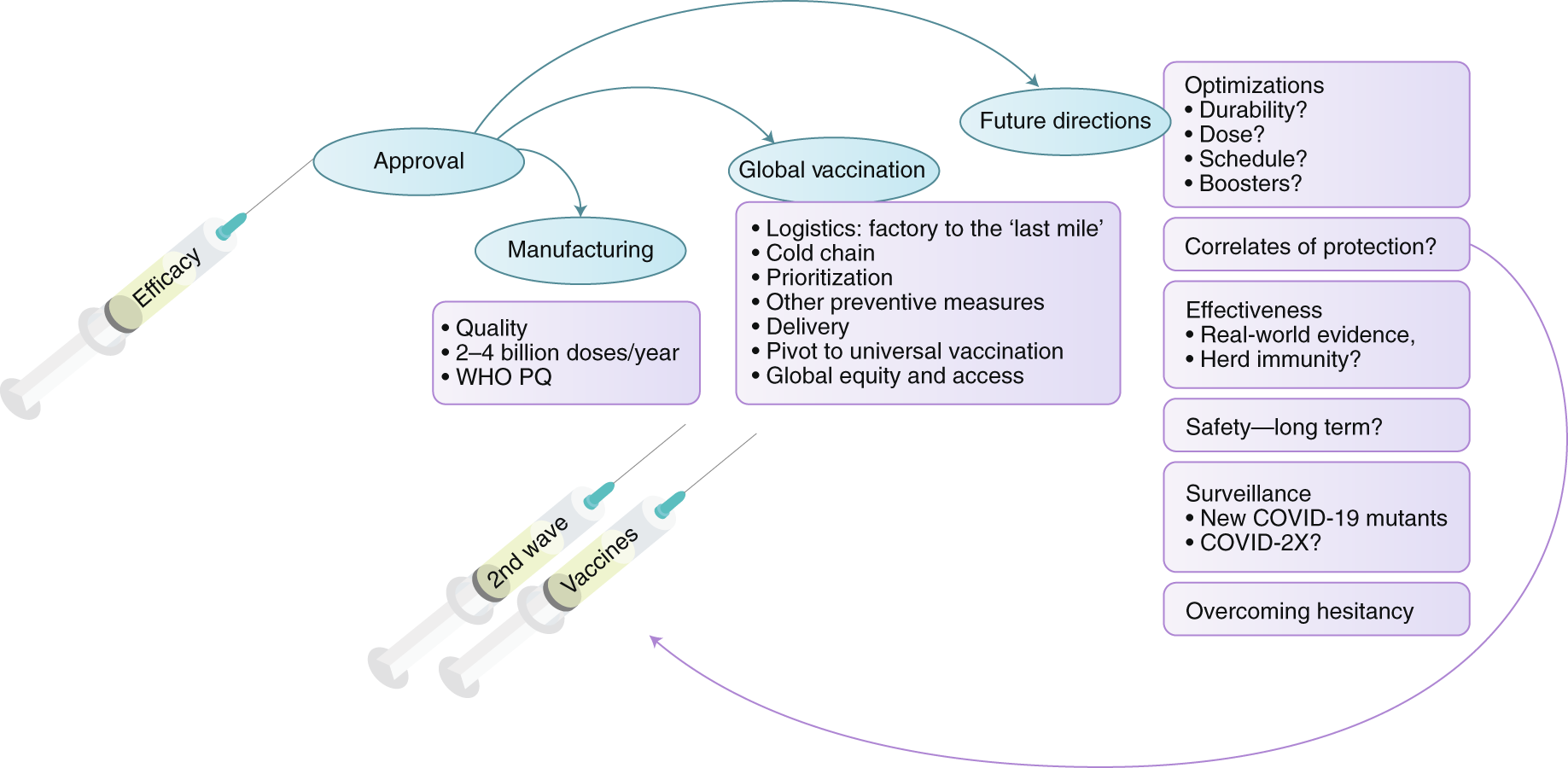
Looking Beyond Covid 19 Vaccine Phase 3 Trials Nature Medicine
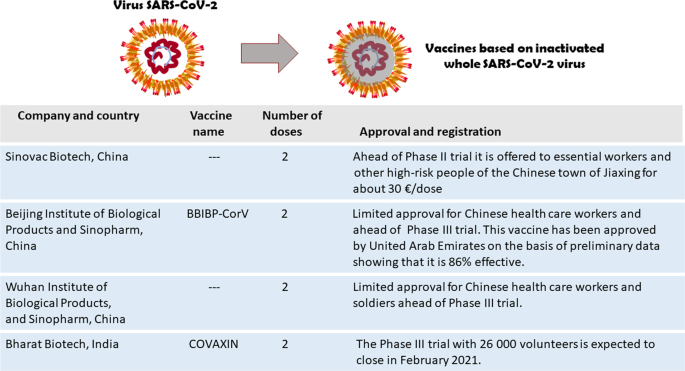
Covid 19 Vaccines Where We Stand And Challenges Ahead Cell Death Differentiation

Hungary Approves Russian Coronavirus Vaccine For Use
Covid What Do We Know About China S Coronavirus Vaccines Bbc News

Safety And Efficacy Of An Rad26 And Rad5 Vector Based Heterologous Prime Boost Covid 19 Vaccine An Interim Analysis Of A Randomised Controlled Phase 3 Trial In Russia The Lancet
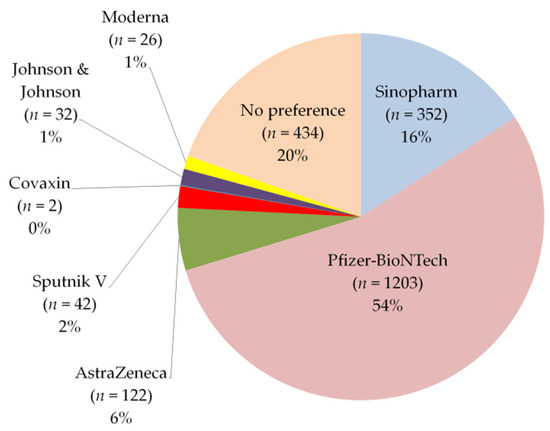
Vaccines Free Full Text Side Effects And Perceptions Following Covid 19 Vaccination In Jordan A Randomized Cross Sectional Study Implementing Machine Learning For Predicting Severity Of Side Effects Html

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn

Safety Tolerability And Immunogenicity Of An Inactivated Sars Cov 2 Vaccine In Healthy Adults Aged 18 59 Years A Randomised Double Blind Placebo Controlled Phase 1 2 Clinical Trial The Lancet Infectious Diseases

Challenges In Ensuring Global Access To Covid 19 Vaccines Production Affordability Allocation And Deployment The Lancet